Research regarding the different properties and potential applications of different chemicals and materials has been the driving force behind the major advances in science and technology that have occurred over the past century. Discovering and measuring the impact that chemicals such as mTBD can have on improving scientific technology is just one example of how small discoveries can make a big change towards improving production industries, increasing efficiency and making current technologies more environmentally friendly. Dr. Daniel Cedarkrantz of Thermtest Europe, had the pleasure of being a part of this research project headed by Aalto University in Helsinki, Finland that has succeeded in measuring and publishing the thermal and transport properties of mTBD (a common catalyst in chemical reactions). This research provides multiple new measurements on mTBD that will likely contribute to its future uses in new technology and industries. Thermtest is delighted to be a part of new and exciting research that has the possibility to open many doors towards the potiential uses of an extremely versatile chemical like mTBD. The article below is focused on the research and discoveries made in the journal referenced below:
Baird, Z.S., Dahlberg, A., Uusi-Kyyny, P. et al. Int J Thermophys (2019) 40: 71. https://doi.org/10.1007/s10765-019-2540-2
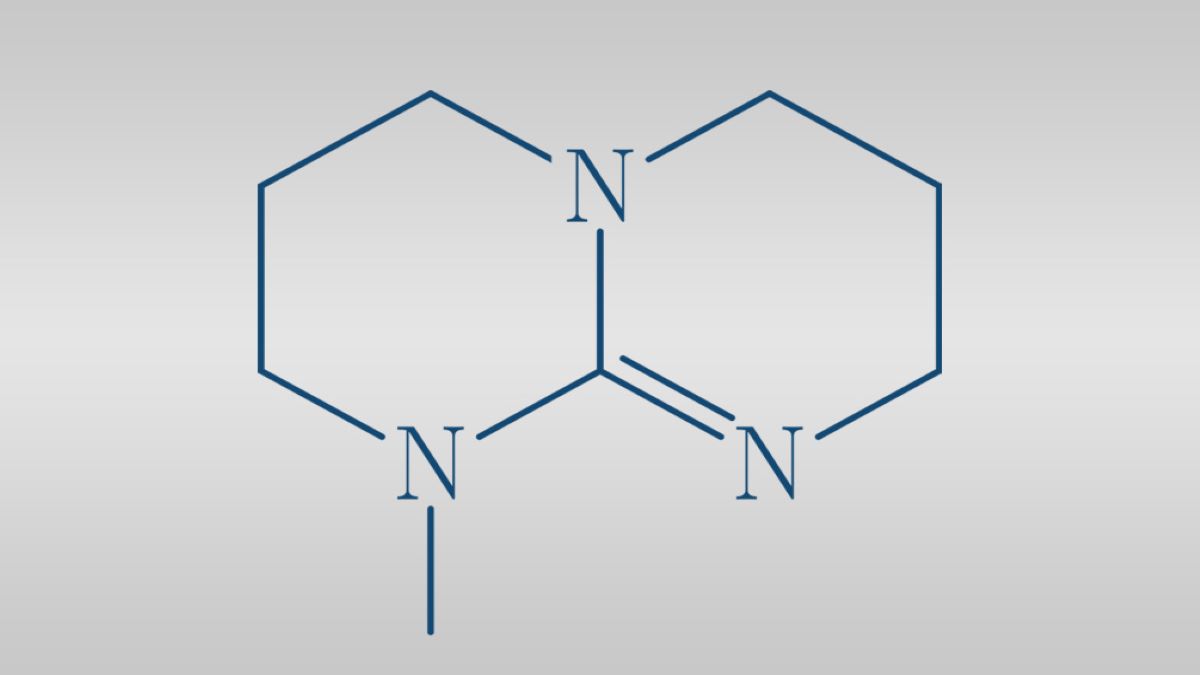
7-Methyl-1,5,7-triazabicyclo[4,40]dec-5-ene (mTBD) is a bicyclic strong guanidine base that is commonly used as catalyst in many chemical reactions. It can form an ionic liquid when mixed with an acid and has been recently studied as a possible chemical to use for carbon dioxide storage. Despite the multiple important industrial uses of mTBD there was no publicly available data on its thermal and transport properties until the research conducted by Baird et al was presented in The International Journal of Thermophysics in 2019. This comprehensive study measured data on multiple properties of mTBD including; the liquid vapor pressure, compressed density, liquid heat capacity, melting properties, liquid thermal conductivity, refractive index, and liquid viscosity of mTBD. The experimental data was then used to further analyze and calculate other properties of mTBD including; enthalpy of vaporization, thermal expansion and isothermal compressibility. A PC-SAFT equation of state based on statistical associating fluid theory was then used to state and compare the thermodynamic properties of mTBD, DBN, DBU and TBG. It is crucial to understand the thermal and transport properties of important superbases such as 7-Methyl-1,5,7-triazabicyclo[4,40]dec-5-ene (mTBD) so their potential uses can be further researched and explored.
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD)](https://thermtest.se/wp-content/uploads/2019/10/backbone-structure-dbu-tbd-mtbd.jpg)
One potential use of mTBD is in carbon dioxide (CO2) capture and storage. There are currently research projects underway to investigate the CO2 capturing properties of ionic liquid superbases that include chemicals such as mTBD. These efficient and fully reversable catch and release CO2 capture technologies use non-volatile ionic liquids. This method provides a safer alternative to current CO2 capture techniques which are based around extremely volatile alkanols or alkylamines (Wang et al, 2010). Carbon dioxide emissions have been increasing exponentially at an alarming rate since the beginning of the industrial revolution. It has become arguably the most significant greenhouse gas relating to climate change that’s is a major product of many anthropogenic practices. The overabundance of CO2 in the atmosphere emphasizes the importance for research to discover a way to store the excess. Using a mixture of alcohol and an amidine or guanidine super base (such as mTBD) can provide safe and efficient carbon dioxide storage.
Three different samples were used to measure the thermodynamic and transport properties of 7-Methyl-1,5,7-triazabicyclo[4,40]dec-5-ene (mTBD). The first was sourced from the University of Helsinski in Finland. The second and third were both sourced from BOC science which is in Shirley, New York. The third sample was distilled for further purity. The three sample were used to ensure that each measurement was accurately representing the chemical properly and the results were precise with little deviation between the three measurements. The thermal conductivity of mTBD was measured using the transient hot wire method. These measurements were obtained using a THW-L2 Liquid Thermal Conductivity Meter from Thermtest.

After each property of mTBD was measured, the values were then compared with three similar chemicals known as 1,1,3,3-tetramethylguanidine (TMG), 1,5-diazabicyclo (4.3.0)non-5-ene (DBN), 1.8-diazabicyclo[5.4.0]undec-7-ene (DBU). DBN and DBU are the strongest available amidine bases that are well established reagents in organic synthesis (Wiley et al. 2009). TMG is a strong non-nucleophilic base for alkylation that is commonly used as a substitute for more expensive bases such as DBU and DBN. The results displayed that mTBD had a higher density and lower vapor pressure compared to the other chemicals.
The research conducted by Baird, et al. has shed light on the numerous thermodynamic and transport properties of 7-Methyl-1,5,7-triazabicyclo[4,40]dec-5-ene (mTBD). All the measurements obtained by this team are now publicly available through literature after being accepted in the July 2019 addition of the Internation Journal of Thermophysics. mTBD is an extremely import industrial catalyst and has the potential to assist in combatting climate change as a potential superbase to use in carbon dioxide storage. With this data now publicly available there is the possibility for additional research to take place on potential uses of the guanidine superbase mTBD.
Baird, Z.S., Dahlberg, A., Uusi-Kyyny, P. et al. Int J Thermophys (2019) 40: 71. https://doi.org/10.1007/s10765-019-2540-2
Wang, C., Mahurin, S., Luo, H., Baker, G., Li, H., & Dai, S. 2010. Reversible and robust CO2 capture by equimolar task-specific ionic liquid-superbase mixtures. Green Chemistry. 12(5). 870-874.
Wiley, J. 2009. Superbases for organic synthesis. Ishikawa, T. John Wiley & Sons, LTD. West Sussex, UK. 321
Images:
Corneille, S., Smet, M., 2014. PLA Architectures: the role of branching. Royal Society of Chemistry. 6. 850-867. DOI: 10.1039/C4PY01572J
Author: Kallista Wilson | Junior Technical Writer | Thermtest








![Thermal and Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4,40]dec-5-ene](https://thermtest.se/wp-content/uploads/2019/10/7-Methyl-157-triazabicyclo4.4.0dec-5-ene-1-150x89.jpg)



